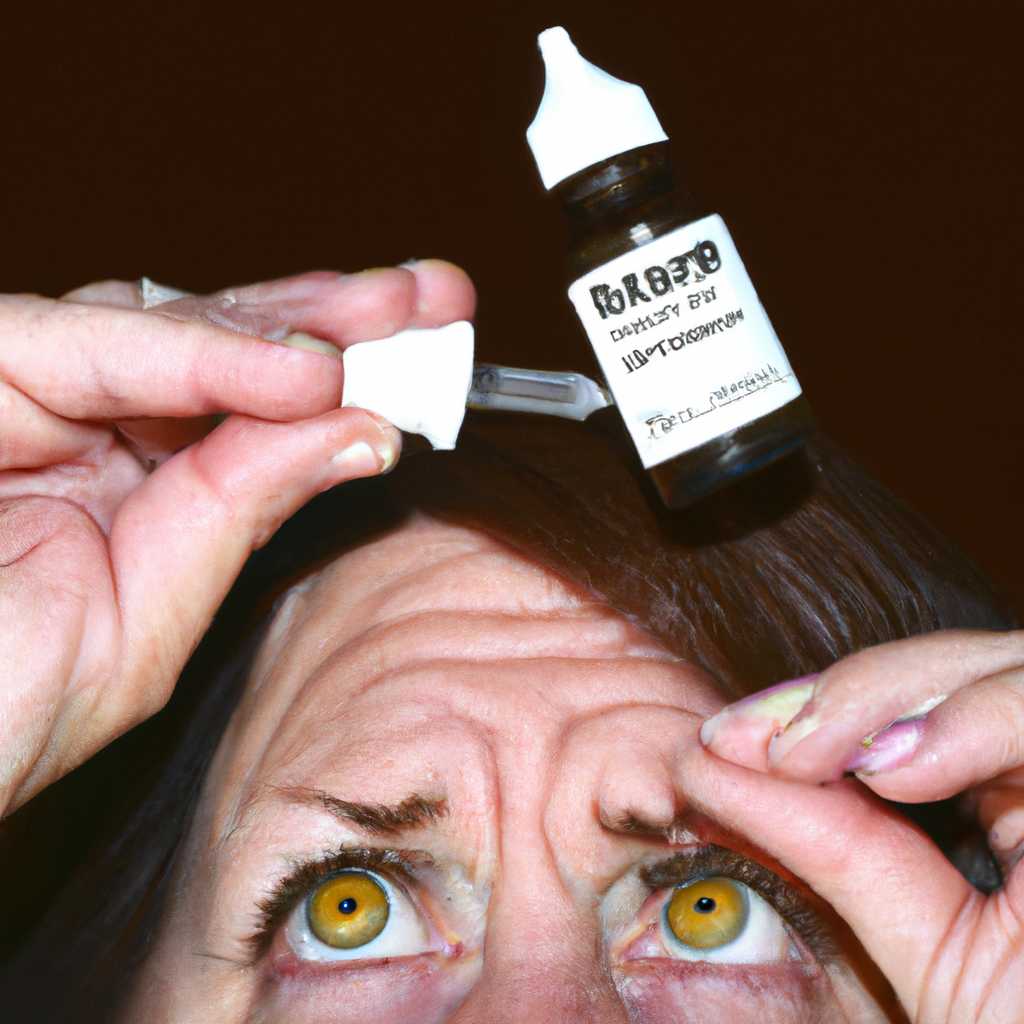
Pseudomonas aeruginosa, an extensively drug-resistant bacterial infection has caused shockwaves across the United States. In February 2021, two brands of artificial tears eyedrops, EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears and Artificial Eye Ointment, were recalled from shelves after being linked to at least 68 cases of Pseudomonas aeruginosa infections. Three patients tragically lost their lives due to the infection, eight reported vision loss and four required the surgical removal of their eyeball.
The CDC noted that infected patients reported using over 10 kinds of artificial tears, with EzriCare artificial tears linked to at least 50 of the infections. Laboratory tests revealed traces of Pseudomonas aeruginosa in the product, which was manufactured in India. As a result, the CDC and the FDA recommended that users cease using both products, and ordered clinicians to not recommend or provide them.
Two patients from Florida, Naples Fire Captain Adam Di Sarro and Cara Oliva, are now suing EzriCare and Delsam Pharma as well as Amazon, which distributed the drops. Di Sarro reported being blinded in one eye, while Oliva had to have her eye removed due to a massive corneal ulcer.
Pseudomonas aeruginosa is a particularly dangerous infection for those with weak immune systems. The bacterium is resistant to most antibiotics and can cause infections such as keratitis, sepsis and respiratory and urinary tract infections. Symptoms of Pseudomonas aeruginosa infection include yellow, green or clear discharge from the eye; eye pain or discomfort; redness of the eye or eyelid; a sensation that there is something in your eye; increased sensitivity to light and blurry vision. (
According to CDC estimates, the United States witnessed about 2,700 deaths related to Pseudomonas aeruginosa and 32,600 cases in hospitalized patients in 2017. This recent outbreak has highlighted the need for improved safety measures, especially in the manufacturing and distribution of artificial tears eyedrops in order to protect the public from potentially life-threatening infections.